
The New Era of Medical Writing and Where AI Meets Expertise in 2026
The landscape of medical writing is rapidly transforming due to artificial intelligence (AI), financial pressures, and changing global regulations. In
Follow Us On:

The landscape of medical writing is rapidly transforming due to artificial intelligence (AI), financial pressures, and changing global regulations. In
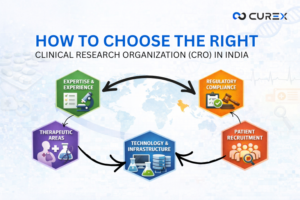
Selecting an appropriate Clinical Research Organization (CRO) is crucial in drug development, particularly within India’s expanding clinical research market. This

In the dynamic realm of pharmaceutical and biotechnology innovation, the journey from drug or therapy concept to market involves intricate

Clinical trial data management (CDM) is essential in pharmaceutical and biotechnology research, enabling the effective handling of extensive data from
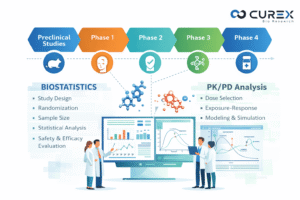
Biostatistics services play a pivotal role in drug development, where the transition from a promising molecule to an approved therapy

In the rapidly evolving pharmaceutical sector, the safety profile of a drug continually develops post-regulatory approval, necessitating ongoing signal detection

1113, Silver Radiance 4 Sarkhej – Gandhinagar Hwy, Chanakyapuri, Ahmedabad, Gujarat 382481

3412 Northwest 110th Way, Coral Springs, FL 33065

203 Ziadodda Cresent, Brampton,Ontario, L6P1T4
© Copyright 2025 Curexbio. All Rights Reserved