Clinical trial data management (CDM) is essential in pharmaceutical and biotechnology research, enabling the effective handling of extensive data from clinical trials that evaluates the safety and efficacy of new treatments. CDM involves the systematic collection, cleaning, validation, and integration of data obtained from clinical trials, aimed at generating results that are reliable, high-quality, and statistically valid. The CDM process includes the design of data collection instruments, particularly electronic Case Report Forms (eCRFs), and emphasizes maintaining data integrity through strict quality control measures and adherence to regulatory standards.
Key activities in Clinical Trials Data Management include:
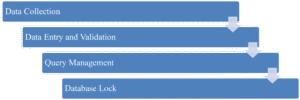
- Data Collection: Gathering information from trial participants via standardized forms.
- Data Entry and Validation: Entering data into secure systems and running checks to identify discrepancies.
- Query Management: Resolving inconsistencies with trial sites.
- Database Lock: Finalizing the dataset for analysis once all queries are resolved.
Modern Clinical Trial Data Management utilizes innovative tools such as Electronic Data Capture (EDC) systems. These advanced systems enhance efficiency by streamlining various processes and significantly minimizing errors when compared to conventional paper-based data collection methods.
Need for Clinical Trial Data Management Critical for Research Success
Poor data quality can significantly hinder the success of clinical trials, highlighting the critical need for effective Clinical Trial Data Management.
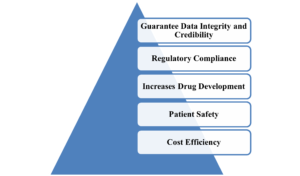
Figure 1: Need for Clinical Trial Data Management
- Guarantee Data Integrity and Credibility : High-quality data is essential for accurate conclusions regarding treatment safety and efficacy, as errors or inconsistencies can undermine results, wasting resources and potentially endangering patients.
- Regulatory Compliance: Bodies like the FDA and EMA necessitate detailed data records for approvals, with robust CDM generating audit-ready datasets that adhere to GCP and CDISC guidelines.
- Increases Drug Development: Clean and validated data accelerates the process from trial completion to market approval by facilitating quicker statistical analysis and decision-making.
- Patient Safety: Timely identification of adverse events through proper data handling is essential for participant protection and informed risk-benefit assessments.
- Cost Efficiency: Preventing data-related delays and rework is crucial for saving expenses in multi-phase trials.
Clinical Data Management transforms raw trial data into dependable evidence, promoting medical innovation.
Partner with CurexBio for Expert Clinical Trial Data Management
As a leading CRO, CurexBio provides comprehensive clinical trial data management solutions designed to expedite the drug development process, ensuring accuracy, compliance, and efficiency across all clinical trial phases.
- Advanced EDC systems with robust security and query management.
- End-to-end support from study design to database lock.
- Integration of cutting-edge technologies for real-time insights and regulatory adherence.
CurexBio combines scientific expertise with operational excellence to assist biotech and pharmaceutical companies in managing complex trials. They provide audit-ready, high-quality data for both early-phase and large-scale global studies, facilitating successful outcomes.
Ready to elevate your clinical research? Visit www.curexbio.com to learn more about CurexBio’s clinical trial data management and full-suite CRO services. Partner with us to turn your innovative therapies into reality, faster, safer, and with unwavering compliance.





