The landscape of medical writing is rapidly transforming due to artificial intelligence (AI), financial pressures, and changing global regulations. In 2026, this pivotal year presents biopharmaceutical companies with both significant opportunities and risks, highlighting the necessity to adopt AI responsibly to enhance development while maintaining scientific integrity and compliance.
At CurexBio, the focus is on fostering a synergistic partnership between human expertise and AI tools. By combining AI with extensive regulatory and therapeutic knowledge, they provide faster, consistent, and strategically aligned medical writing services, emphasizing human accountability.
Table of Contents
ToggleThe 2026 Landscape: Forces Reshaping Medical Writing
The transformation is influenced by the convergence of multiple strong trends such as:
- Technological Transformation: Large Language Models (LLMs) and intelligent authoring systems are now integral tools that enhance drafting speed and aid in complex tasks such as literature synthesis and consistency verification. Regulatory agencies are also investigating the use of AI to improve their review processes, indicating a growing acceptance of AI in document preparation.
- Amplifying Economic Pressure: With limited research funding and increasing development costs, sponsors are pushing for faster deliverables and smaller teams, driving the rapid adoption of AI to reduce costs and accelerate timelines.
- Increasing Regulatory & Geopolitical Intricacy: Clinical development is becoming increasingly complex, incorporating adaptive trials and new techniques such as gene therapies. The geopolitical landscape and the rise of Asian research hubs require documentation that is globally competent and capable of addressing various regulatory frameworks.
The Changing Dynamics of Medical Writing in 2026
| Document Type | Essential AI Opportunity | Key Risk to Mitigate | Critical Compliance Need |
|---|---|---|---|
| Clinical Study Reports (CSRs) | Automated data synthesis and drafting can reduce turnaround time by up to 40%. | Loss of nuanced data interpretation and subtle inconsistencies. | Full traceability with mandatory human sign-off on all conclusions. |
| Protocols & Investigator Brochures | Rapid creation of structured first drafts improves feasibility assessments. | Over-reliance on templated text may weaken study-specific strategy. | Validation against latest regulatory and ICH guidelines. |
| Regulatory Submission Documents | Efficient population of CTD modules ensures consistency across large dossiers. | Incorrect references leading to critical compliance failures. | Rigorous expert review to ensure audit-ready documentation. |
| Medical Affairs & Scientific Communications | Rapid literature reviews and slide generation for timely scientific exchange. | Inappropriate tone or messaging misaligned with the target audience. | Clear AI governance with mandatory medical review for external content. |
AI’s Practical Applications for Modern Medical Writing
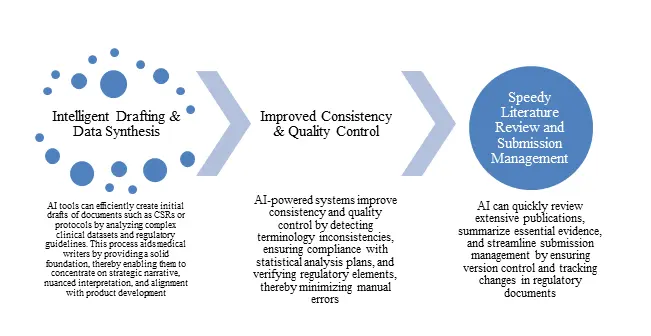
AI is transforming the writing process by serving as a copilot that manages administrative tasks, enabling writers to concentrate on more critical, judgment-based activities.
Figure 1: Practical Applications for Modern Medical Writing
Navigating the Compliance Imperative and Mitigating Risk highlights the misconception that AI reduces oversight in medical writing, emphasizing the opposite. With 2026 anticipated as “the year of governance,” health systems and sponsors are prioritizing formal frameworks for responsible AI usage. The prevalence of “shadow AI” , the unauthorized use of public AI tools poses considerable risks to data security, compliance, and document integrity.
Key compliance pillars for 2026 include:
- Human’s Accountability: Regulatory agencies assert that accountability for submissions lies with humans, emphasizing that AI serves as an assistive tool rather than a substitute for expert judgment. All AI-generated outputs are required to go through thorough expert review and validation.
- Transparency & Audit Trails: Regulators and sponsors now require not only the final document submission but also clear documentation detailing when, where, and how AI was utilized in the writing process. This includes the ability to reproduce and justify decisions made during that process.
- Data Security: Using generic, cloud-based AI models significantly risks leaking confidential clinical data or intellectual property. It is essential to utilize secure, validated, and purpose-built systems within a controlled governance environment.
CurexBio Approach in Medical Writing:
The CurexBio Approach emphasizes the use of AI as a force multiplier in medical writing services, enhancing the team’s expertise rather than serving as a shortcut. This integration of advanced AI within a human-centric framework aims to provide exceptional value in a complex environment.
- Speed and Quality: AI enhances the drafting process and routine checks, allowing experts to concentrate on strategic quality, nuance, and compliance, resulting in faster timelines without compromising the required strategic quality for approval.
- Lack of inconsistency: AI tools create consistent terminology and data presentation across thousands of document pages, ensuring submission-ready consistency that minimizes regulatory questions and delays.
- Guidelines assurance: Navigating evolving global regulations, our AI systems are continuously updated with the latest guidelines from FDA, EMA, ICH, and other agencies, ensuring that documents are based on current regulatory intelligence.
- Cost analysis: In a competitive landscape, the efficient use of AI enables optimized resource allocation, offering both cost control and competitive value. The focus is on delivering high-efficiency services that ensure budget compliance without hidden risks.
Our process emphasizes collaboration with clinical, regulatory, and statistical teams to produce scientifically sound and submission-ready documents, including Clinical Trial Protocols and Regulatory Submission Documents.
Looking towards the future, the partnership between humans and AI in medical writing will evolve, requiring professionals to prioritize continuous upskilling and ethical AI stewardship. We, CurexBio a medical writing CRO, as a resource for navigating AI-driven medical writing to expedite regulatory milestones.





