In clinical trials, data integrity is crucial, yet even meticulously designed studies can encounter issues due to inconsistencies or gaps in the collected data. Query management plays a critical role in addressing these problems, serving as a mechanism to catch and rectify errors before the final analysis. This blog explores the complete life cycle of a query—from identification to resolution—while highlighting common areas where time is lost, complicating what could be a straightforward correction. For those involved in trial operations or delivering end to end clinical data management services, grasping this cycle is essential for optimizing workflows and enhancing data quality.
Table of Contents
ToggleWhy Query Management Matters in Clinical Trials
At its core, query management serves to enhance communication among data teams, monitors, and site staff, facilitating the identification and rectification of erroneous data. Queries act as focused inquiries that expose missing information, irregularities, or significant errors, which are crucial in ensuring the reliability of clinical trials. Neglecting query management can lead to unreliable results, risking regulatory compliance under ICH E6(R3) Good Clinical Practice guidelines.
Essentially, queries are integral to maintaining data integrity, completeness, and credibility, playing a vital role in overall data cleaning initiatives that help uphold trial progress and compliance. However, the management of such queries requires careful execution; ineffective handling can lead to increased costs, resource strain, and delays in timelines. As clinical trials become increasingly complex with the integration of electronic data capture (EDC) systems, proficiency in query management is essential for anyone involved in comprehensive clinical data management services.
- Breaking Down the Query Life Cycle
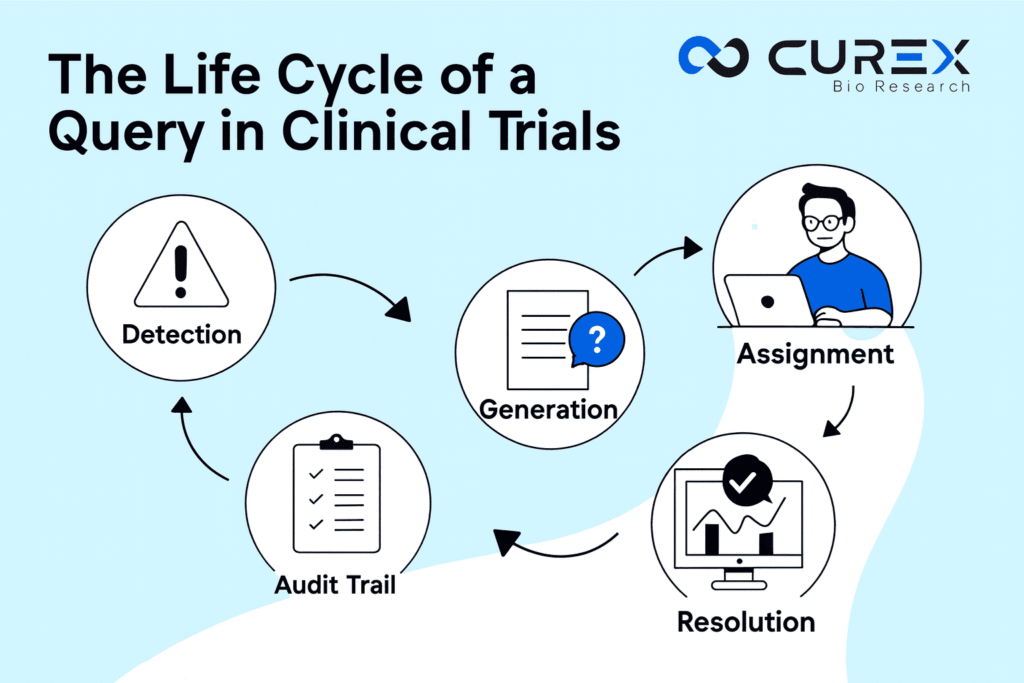
The journey of a query isn’t random—it’s a structured workflow with distinct phases. Here’s how it typically unfolds, reimagined from standard practices in the field:
- Detection: Spotting the Red Flags
Everything begins with the identification of issues in data management, which may include mismatched dates, out-of-range laboratory values, or empty fields requiring attention. This detection is facilitated through two primary methods: automated checks integrated within Electronic Data Capture (EDC) systems that alert users to problems immediately during data entry, and manual reviews conducted by data managers, Clinical Research Associates (CRAs), or Clinical Trial Managers (CTMs). The overarching objective is to identify discrepancies early in the process to prevent more significant complications in the future.
Automated checks must be finely tuned during study setup to avoid missing subtle issues, which can lead to increased manual troubleshooting. Inefficient detection may overwhelm teams with queries and slow down progress.
- Generation: Crafting the Right Question
Once a problem is flagged, it’s time to create the query itself. Effective communication requires concise and precise messages that clearly identify issues and specify necessary actions. Vague inquiries lead to confusion and extended clarification processes, causing significant time loss due to the “ping-pong” effect of incomplete responses.
- Assignment: Directing It to the Right Hands
In clinical trials, queries are assigned to the relevant personnel, typically site staff, investigators, or coordinators, but may also involve coders or monitors for specific concerns. This allocation often occurs digitally in EDC platforms. However, delays can arise from misrouting or unclear responsibilities, exacerbated by time zone differences and language barriers in global trials, leading to lengthy waiting periods if assignments end up in incorrect inboxes.
- Monitoring: Keeping Tabs on Progress
Queries require proactive management and oversight, typically by data managers or Clinical Trial Managers (CTMs), who utilize dashboards to monitor aging queries and encourage timely responses. Inadequate monitoring tools can result in unresolved queries, with industry data showing average resolution times between 23 to 52 days, and in some cases, weeks or months. This phase often intensifies the “ping-pong” effect, where incomplete responses lead to reassignments and prolonged follow-ups.
- Resolution: Closing the Loop
When a site updates data or provides explanations, the query owner reviews the response. If acceptable, the query closes; if not, further revisions are required. Multi-round clarifications frequently occur, particularly with endpoint data. A study of a cardiovascular trial revealed that 21% of queries necessitated multiple submissions, leading to prolonged closure and potential database lock delays. Strong clinical data management services help minimize these repeated cycles by improving clarity and consistency in query handling.
- Audit Trail: Documenting It All for Posterity
No query life cycle is complete without a solid record, as the audit trail captures details such as who raised a query, the timing, actions taken, and resolutions. This documentation is essential for traceability in Good Clinical Practice (GCP) and identifying patterns like recurring errors. Insufficient documentation can lead to audits or inspections, resulting in retroactive fixes that interrupt progress. However, thorough documentation can highlight trends and help prevent future issues.
Queries in clinical trials often bounce between teams, causing delays and inefficiencies. With 0.14–0.4 queries per CRF page, mid-sized trials can generate thousands, costing up to $200 each, predominantly yielding no changes. This creates significant pressure on sites managing these queries alongside patient care, while sponsors and CROs focus on compliance. Effective clinical data management services can significantly reduce these inefficiencies by optimizing processes and minimizing unnecessary queries.
Key Challenges:
- Redundant or Irrelevant Queries: Over-configured automated checks spam sites with low-impact flags.
- Communication Gaps: Unclear phrasing or lack of context leads to incomplete responses.
- Resource Strain: High volumes near database lock force overtime, inflating budgets.
- Timeline Delays: Slow resolutions postpone analyses and submissions, potentially costing thousands per day.
In one Phase I study review, only 28-40% of queries fixed data, underscoring the inefficiency.
Best Practices to Speed Things Up
Adopt these strategies to overcome delays:
- Standardize Everything: From data entry guidelines to query templates, consistency cuts errors upfront.
- Prioritize Smartly: Triage queries by impact—focus on critical endpoints first.
- Train and Collaborate: Cross-team sessions align everyone, reducing misunderstandings.
- Leverage Metrics: Track turnaround times, volumes, and rates to refine processes and strengthen overall clinical data management services outcomes.
The future of technology highlights the role of AI, NLP, and predictive analytics in automating detection and generation, enabling real-time monitoring and decentralized trials that promise quicker resolutions. This shift emphasizes proactive prevention over reactive fixes, significantly reducing query volumes and strengthening modern clinical data management services.
Wrapping Up: Turning Queries into a Strength
Mastering the query life cycle is essential for efficient and high-quality clinical trials that improve medicine. Reducing time spent in communication allows teams to concentrate on generating reliable data and enhancing patient outcomes. Organizations can benefit from partnering with providers of comprehensive end to end clinical data management services to streamline the entire process, from query detection to resolution.
Curex Bioresearch’s clinical data management team enhances the entire process through automation, risk-based prioritization, and real-time monitoring, ensuring full GCP-compliant audit trails. By using automated tools and predictive analytics, they expedite discrepancy resolution, reduce site burden, and maintain data integrity. Interested in evolving query management? Submit an RFI here.