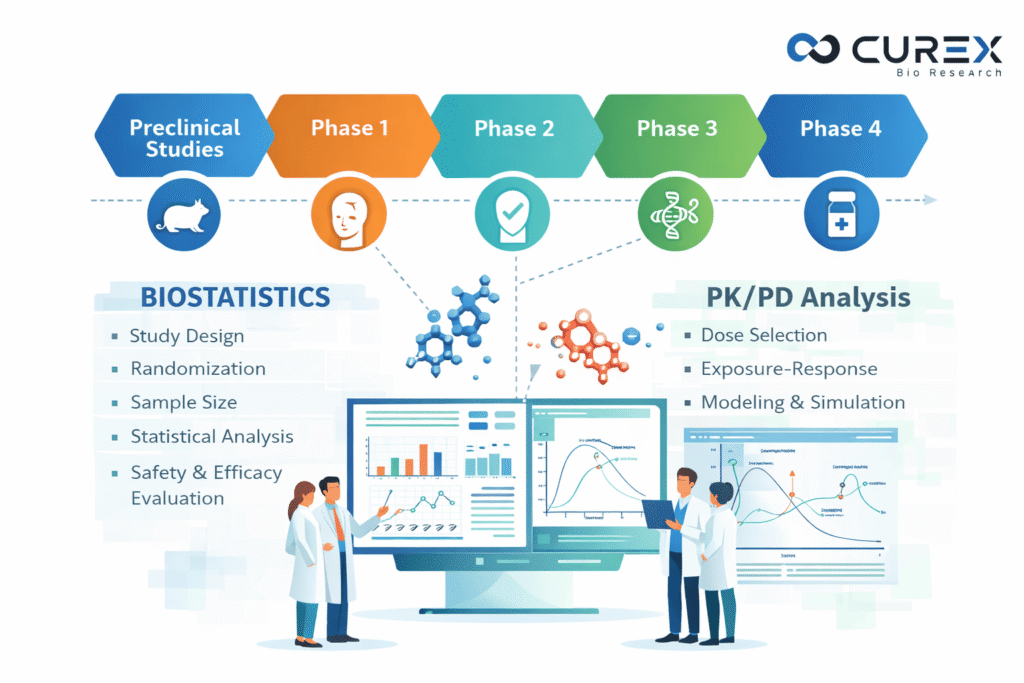
Biostatistics services play a pivotal role in drug development, where the transition from a promising molecule to an approved therapy requires rigorous evidence generation for safety and efficacy. Central to this process are biostatistics and pharmacokinetic/pharmacodynamic (PK/PD) analysis, which convert raw clinical data into actionable insights for informed decision-making in clinical trials. For researchers, pharmaceutical professionals, and stakeholders involved in the drug approval process, understanding these disciplines is essential. Additionally, CurexBio, a global Clinical Research Organization (CRO), provides comprehensive biostatistics services in clinical research to strengthen and accelerate drug development programs.
-
The Phases of Drug Development and Clinical Trials
Drug development involves a multi-stage process, where clinical trials are divided into phases to evaluate new therapies systematically.
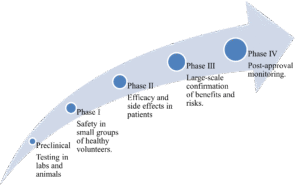
Figure 1: CurexBio’s Clinical Trial Process
Biostatistics and pharmacokinetics/pharmacodynamics (PK/PD) are essential for making data-driven decisions throughout various phases.
-
Biostatistics in Clinical Trials
Biostatistics uses statistical methods to analyze biological and medical data, ensuring that clinical trial results are reliable, unbiased, and scientifically interpretable. It supports critical activities such as study design, sample size determination, randomization, data analysis, and interpretation of safety and efficacy outcomes. These statistical approaches are aligned with regulatory expectations outlined by the U.S. Food and Drug Administration, as detailed in the FDA guidance on statistical principles for clinical trials.
Key roles include:

Figure 2: Biostatistics Application in Clinical Trial
Visualizations are essential in biostatistics, exemplifying how data is effectively presented in clinical trials. Without robust biostatistics, trials risk flawed conclusions, wasted resources, or regulatory rejection.
-
PK/PD Analysis:
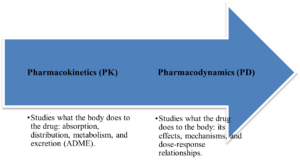
Figure 3: CurexBio’s PK/PD modeling
-
Role of PK/PD analysis in drug development
PK/PD modeling associates drug exposure with its therapeutic effect, allowing for predictions of optimal dosing strategies.
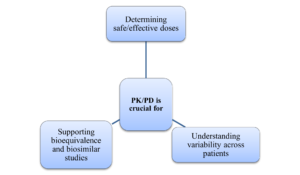
Figure 4: Classic PK/PD curves illustrate these relationships
-
Biostatistics and PK/PD Essential in Drug Development
These tools are essential for reducing risks, optimizing designs, and providing necessary evidence for regulatory approvals in pharmaceutical development. With robust pharmacokinetic/pharmacodynamic (PK/PD) data, predictions about human responses can be made from preclinical studies, while biostatistics ensures that statistical significance is meaningful in clinical contexts. The combined use of these methodologies effectively lowers attrition rates, accelerates timelines, and ultimately facilitates the faster delivery of safer therapies to patients.
CurexBio Supports Your Clinical Trials
CurexBio with facilities in India, the USA, and Canada, offers comprehensive clinical research services. Their expertise encompasses critical areas such as biostatistics and pharmacokinetics/pharmacodynamics (PK/PD) analysis.
Our offerings include:

Figure 5: CurexBio Biostatistics Services
With extensive knowledge in various therapeutic areas, CurexBio provides biostatistics services that guarantee compliance, precision, and innovation, facilitating the process from protocol development to regulatory submission. We offers top-tier biostatistics and pharmacokinetics/pharmacodynamics (PK/PD) expertise, aimed at enhancing clinical trials.