Selecting an appropriate Clinical Research Organization (CRO) is crucial in drug development, particularly within India’s expanding clinical research market. This decision profoundly impacts the trial’s timeline, cost, compliance, and overall success. As the Indian CRO market is anticipated to experience significant growth, the abundance of available options adds to the complexity of making this essential choice. However CurexBio, a clinical trial CRO in India offered services from clinical trial design to implementation with the backhand support of regulatory and pharmacovigilance.
India is a Prime Hotspot for Clinical Research
India has established itself as a global hub for clinical trials, attributable to its unique advantages that provide a compelling case for collaboration with a skilled clinical research organization in the country.
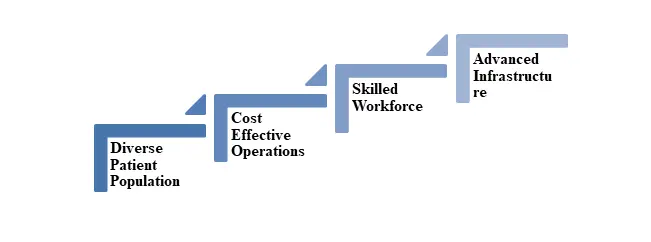
Figure 1: India capability for Clinical Research
- Diverse Patient Population: refers to a wide array of ethnicities and backgrounds within a treatment-naïve group, creating a substantial genetic variety that supports the collection of comprehensive and broadly applicable clinical data.
- Cost Effective Operations: High-quality research can be performed at a substantially reduced cost in comparison to Western nations, while still adhering to international standards.
- Skilled Workforce: India boasts a substantial and skilled workforce comprising a vast pool of highly qualified professionals in clinical trial management, along with scientists and English-speaking clinicians.
- Advanced Infrastructure: The country possesses contemporary research facilities, a network of hospitals, and a robust IT foundation that facilitates advanced data management and trial operations.
Key Factors for Selecting the Best CRO in India
When evaluating potential partners, key dimensions must be considered, as outlined in the accompanying table that details how CurexBio meets these standards.
| Evaluation Factor | What Should You Look for | CurexBio Aligns |
| Therapeutic & Regulatory Expertise | Deep experience in therapeutic areas such as oncology and rare diseases is essential. It is important to have the ability to navigate complex regulatory pathways with agencies like the Drug Controller General of India (DCGI), USFDA, and EMA. When evaluating potential partners, critical dimensions must be considered. | Specializes in end-to-end clinical development from Phase I to IV, ensuring protocol designs and operations meet specific therapeutic needs while maintaining rigorous compliance with ICH-GCP and global standards.
|
| Quality & Data Integrity | Integrated, end-to-end services include protocol writing, site selection, data management, biostatistics, and medical writing for seamless trial execution.
|
Employs a clinical data management system designed for precision and compliance with 21 CFR Part 11 and CDISC, featuring real-time data cleaning and validation to maintain audit-ready data integrity throughout the trial lifecycle.
|
| Operational Excellence & Mindset | A partner committed to timely delivery and proactive problem-solving, prioritizing quality over volume and treating your trial as a priority rather than just a project.
|
Adopts a philosophy of prioritizing high-quality, time-bound completion of projects while fostering high-trust partnerships with sponsors to encourage repeat business.
|
| Complete On demand services | Integrated services encompass protocol writing, site selection, data management, biostatistics, and medical writing, facilitating seamless execution of trials.
|
Offers a comprehensive range of CRO services in India, such as clinical trial management, pharmacovigilance, scientific writing, and data management, functioning as a strategic partner.
|
| Technological Innovation | Adoption of modern tools such as advanced Electronic Data Capture (EDC) systems, risk-based monitoring, and digital platforms is aimed at enhancing patient recruitment and trial efficiency. | Integrates advanced technologies and smart study designs to enhance efficiency and patient-centricity, while its data management platform promotes streamlined workflows and real-time visibility for sponsors. |
Partnering for Success in a Growing Market
The clinical trial management landscape in India is rapidly evolving due to technological advancements, a stronger regulatory framework, and growing demand for specialized services in biotechnology and genomics. In this competitive market, selecting a partner involves more than just evaluating services; it requires finding a CRO that offers deep local expertise, adheres to global operational standards, acts as a proactive strategic advisor, and is dedicated to maintaining data integrity and patient safety.
CurexBio exemplifies a partner-led model, emphasizing scientific expertise, regulatory excellence, and a strong commitment to quality. We positions our self not merely as a service provider but as a dedicated ally in facilitating clinical development from concept to market. For those interested in enhancing their clinical programs through a focused, quality-driven partnership, CurexBio invites you to visit our website www.curexbio.com to discover our wide range of services and connect with our team of experts.