In the dynamic realm of pharmaceutical and biotechnology innovation, the journey from drug or therapy concept to market involves intricate scientific validation, regulatory adherence, and coordinated efforts across several phases. End-to-end clinical research services, provided by Contract Research Organizations (CROs), play a crucial role in this process. These services minimize risks, speed up timelines, and ensure patient safety by offering comprehensive solutions, from early preclinical studies to clinical trial management and post-marketing surveillance. We at CurexBio, a clinical research service company, exemplify trusted partners in this sector, delivering integrated expertise to assist biotech, pharmaceutical, and medical device firms in transforming innovative ideas into effective treatments.
End-to-End Clinical Research Services
End-to-end clinical research services provide comprehensive support throughout the drug or device development lifecycle, unlike niche providers that focus on specific areas. Full-service CROs enable sponsors to either outsource the entire process or choose modular components for greater flexibility.
Key phases typically include:
This integrated approach promotes continuity, minimizes handoff errors, and adheres to global standards, including ICH-GCP, FDA, EMA, and CDSCO guidelines.
The Journey: From Preclinical to Advanced Clinical Trials
Preclinical Studies: Laying the Foundation
Preclinical evaluation of a compound’s risks and benefits precedes human testing, involving in vitro assays, animal models for toxicology, safety pharmacology, and pharmacokinetics/pharmacodynamics (PK/PD) studies. A reliable Contract Research Organization (CRO) guarantees that these studies comply with Good Laboratory Practices (GLP) and yield substantial data for Investigational New Drug (IND) applications.
Transition to Clinical Development
Human trials commence after preclinical data is validated, facilitated by end-to-end services that include protocol development, regulatory strategy and submissions, and obtaining ethical approvals (IRB/IEC).
Phase I-IV Clinical Trial Management
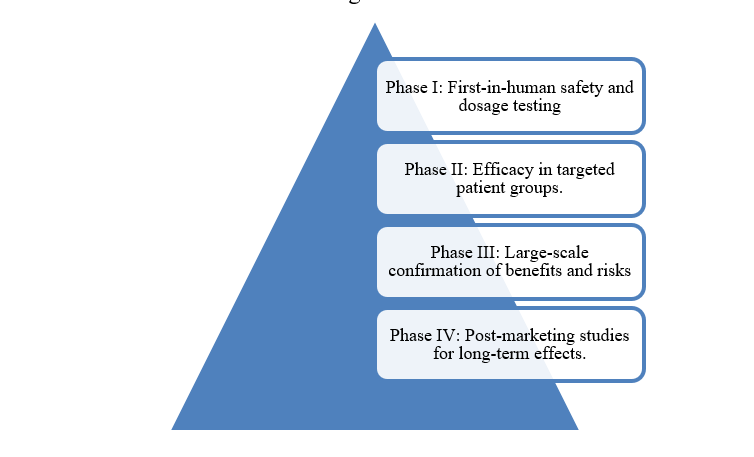
Phases of clinical trial
Advanced management encompasses site monitoring, patient recruitment strategies, real-time data oversight, and adaptive trial designs for efficient complexity handling.
Additional Core Services
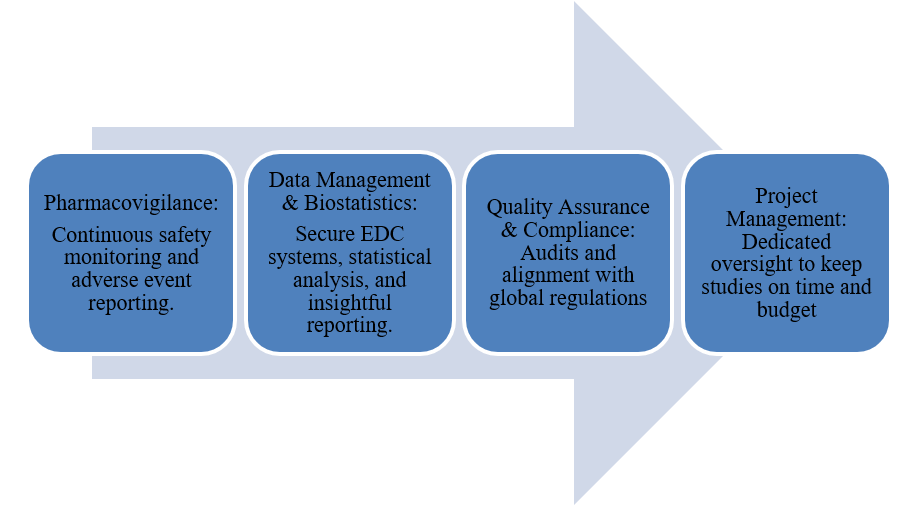
CurexBio integral services
Partnering with a dedicated CurexBio offers distinct advantages:
CurexBio’s clinical research expertise
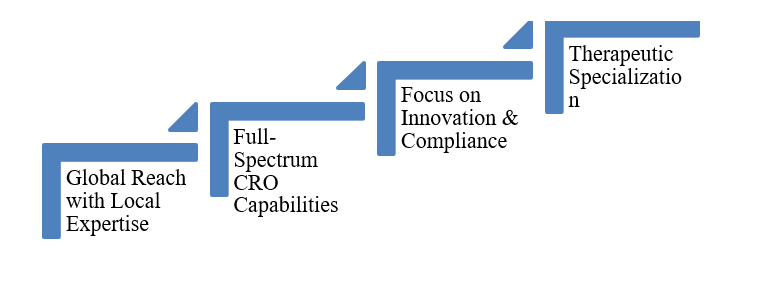
CurexBio’s team of experts delivers precision, integrity, and collaboration, making them an ideal partner for companies seeking reliable end-to-end support.
The evolving landscape of clinical research faced challenges such as increasing costs, demanding regulations, and the necessity for diverse patient populations. It emphasizes the need of experienced CROs like CurexBio, which enable sponsors to prioritize innovation while ensuring trials remain efficient, ethical, and successful.
Explore CurexBio’s services on www.curexbio.com for a more effective transition from preclinical discovery to market success, promoting the core message of transforming scientific advancements into practical solutions through clinical trials.





