In the fast-evolving world of healthcare and pharmaceuticals, clear and compliant communication is not just a necessity—it’s a strategic advantage. Two specialized fields that often come into focus are Regulatory Medical Writing and Scientific Writing. While they may sound similar, they serve distinct purposes, audiences, and regulatory needs.
At CUREXBIO, one of the leading providers of medical writing services in India, we offer expert support in Medical and Scientific Affairs to help organizations navigate these critical domains with precision and compliance. Let’s break down the key differences.
Table of Contents
ToggleWhat is Regulatory Medical Writing?
Regulatory Medical Writing is a specialized discipline focused on creating documents that support the approval of drugs, medical devices, and other healthcare products by regulatory authorities such as the FDA or EMA.
This type of writing is highly structured, precise, and governed by strict guidelines such as ICH-GCP. It requires a deep understanding of clinical trial lifecycles, regulatory standards, and the ability to present complex data clearly and accurately.
Common documents include:
• Clinical Study Reports (CSRs)
• Investigator Brochures (IBs)
• New Drug Applications (NDAs)
• Common Technical Documents (CTDs)
• Pharmacovigilance and safety reports
Regulatory writers must ensure that every document is compliant, consistent, and tailored to the expectations of health authorities.
What is Scientific Writing?
Scientific Writing, on the other hand, is broader in scope and audience. It involves communicating scientific and medical information to a variety of readers—from healthcare professionals and researchers to patients and the general public.
The goal is to make complex information accessible, engaging, and informative. Scientific writers often work on:
• Research articles for peer-reviewed journals
• Patient education materials
• Clinical guidelines
• White papers, blogs, and digital content
• Medical marketing and training materials
Unlike regulatory writing, scientific writing is more adaptable in tone and style, depending on the target audience.
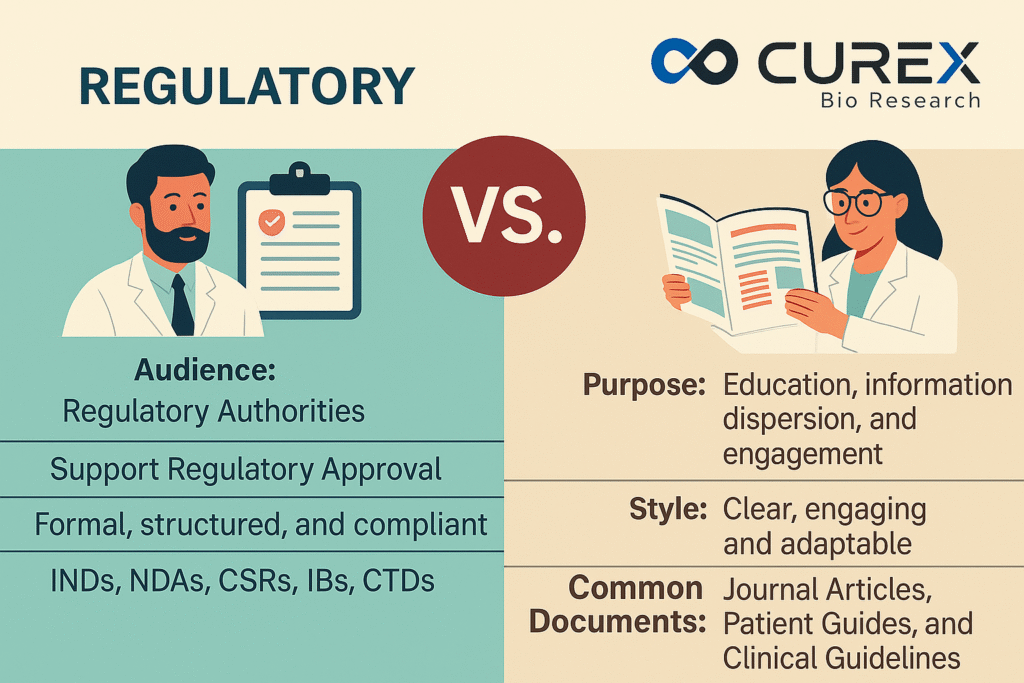
Major Difference at a Glance
| Aspect | Regulatory Medical Writing | Scientific Writing |
|---|---|---|
| Audience | Regulatory Authorities | Healthcare Professionals, Patients, and Public |
| Purpose | Support Regulatory Approval | Education, information dispersion, and engagement |
| Style | Formal, structured, and compliant | Clear, engaging, and adaptable |
| Common Documents | INDs, NDAs, CSRs, IBs, CTDs | Journal Articles, Patient Guides, and Clinical Guidelines |
Both Are Essential
In today’s competitive landscape, pharmaceutical and biotech companies must excel in both areas. Regulatory writing ensures that products meet safety and efficacy standards and gain market approval. Scientific writing helps disseminate knowledge, support medical education, and build trust with stakeholders.
How CUREXBIO Can Help
At CUREXBIO, we provide comprehensive medical writing services in India backed by deep expertise in Medical and Scientific Affairs. Our team of seasoned writers and regulatory experts can support you with:
• Regulatory Document Preparation – From protocols to submission-ready dossiers
• Scientific Communication – Journals, patient materials, CME content, and more
• Medical and Scientific Strategy – Ensuring your messaging is both compliant and compelling
Whether you’re preparing for a regulatory submission or launching a new educational campaign, we provide the precision, clarity, and strategic insight you need.
Ready to elevate your medical and scientific communications?
Contact CUREXBIO today to learn how our medical writing services in India can support your goals in regulatory and scientific excellence.
Get in touch with our team to discuss your needs.