Data Management Systems play a pivotal role in the high-stakes realm of pharmaceutical development, where the urgency to bring a new drug to market transcends financial considerations and directly impacts lives. The drug development process is characterized by its complexity, substantial costs, and often spans over a decade. Within this intricate journey, data management emerges as a critical element that significantly affects both the speed of development and the overall success of new treatments. Traditional strategies for collecting, cleaning, and analyzing data from clinical trials have become inadequate in the face of growing demands. As a result, the shift towards advanced Data Management Systems (DMS) is transforming the industry landscape. These modern systems are essential for streamlining operations, leading to shorter timelines for drug development while simultaneously improving the quality and integrity of the data collected. This evolution in data management practices plays an instrumental role in ensuring timely and effective drug delivery to patients in need.
Table of Contents
ToggleThe Data Gridlock in Traditional Drug Development
In the context of traditional drug development, a significant issue arises from the data bottleneck created by the fragmentation and silos of clinical data across various systems and formats. This problem encompasses different sources of data, including Electronic Data Capture (EDC) systems, clinical laboratory results, and patient-reported outcomes. Such disintegration of data hinders efficient analysis, impeding the overall progression of clinical trials and the drug development process.
- Manual Errors: Time-consuming manual data entry and reconciliation increase the risk of inaccuracies.
- Delayed Queries: Identifying and resolving data discrepancies becomes a slow, back-and-forth process.
- Lack of Real-Time Insight: Sponsors cannot make proactive, data-driven decisions, leading to costly protocol amendments or even trial failures.
How Advanced Data Management Systems Accelerate Development
Modern integrated Data Management Systems (DMS) address and dismantle traditional barriers that impede data flow, resulting in a streamlined, automated, and intelligent data pipeline. This transformation significantly influences timelines across various processes by enabling efficient data handling, integration, and accessibility. As organizations adopt these systems, they can expect improved decision-making and faster response times, ultimately enhancing productivity and operational capabilities.
Centralized and Standardized Data Collection
Advanced systems provide a single source of truth for all trial data. By standardizing data formats and employing centralized EDC systems, they eliminate inconsistencies from the start. This reduces the time spent on data cleaning and harmonization by weeks or even months.
Automated Query Management
Intelligent systems can automatically flag discrepancies or missing data in real-time, triggering instant queries to sites. This automation drastically cuts down the query resolution cycle, which is one of the most prolonged phases in data management.
Real-Time Analytics and Reporting
With cloud-based platforms, stakeholders can access clean, curated data in real-time. This enables:
Faster Go/No-Go Decisions: Early trends in efficacy or safety can be identified quickly.
Proactive Risk Management: Potential issues are spotted before they derail a trial.
Streamlined Regulatory Submissions: Ready-to-analyze datasets expedite the creation of Clinical Study Reports (CSRs) and other submission documents for regulatory authorities.
Enhanced Integration Capabilities
A robust Data Management System integrates seamlessly with other platforms—such as safety databases, interactive response technology (IRT), and electronic health records (EHR). This interoperability ensures a continuous and error-free flow of information, eliminating manual transfer delays.
Key Procedures in an Advanced Data Management Workflow
Implementing a cutting-edge system involves a structured, procedural approach:
Table 1: Process of Data Management at CUREXBIO
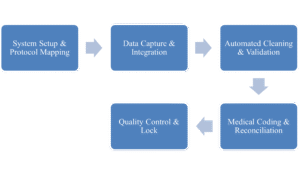
- System Setup & Protocol Mapping: The trial protocol is translated into electronic case report forms (eCRFs) and validation checks within the EDC system.
- Data Capture & Integration: Data is captured directly from sites and integrated from various sources (labs, wearables, etc.) into a central repository.
- Automated Cleaning & Validation: Pre-programmed edit checks run continuously, identifying errors without manual intervention.
- Medical Coding & Reconciliation: Adverse events and medications are automatically coded using standardized dictionaries (like MedDRA and WHODrug), and discrepancies are flagged for review.
- Quality Control & Lock: Continuous monitoring ensures data quality, leading to a faster and more reliable database lock—the critical final step before analysis.
CUREXBIO: Your Partner in Accelerating Drug Development with Smart Data Management
At CUREXBIO, we understand that efficient data management is the backbone of successful and timely drug development. We offer end-to-end Data Management System solutions designed to streamline your clinical trials from start to finish.
Our services include:
- Implementation of State-of-the-Art EDC Systems: We deploy user-friendly, compliant systems tailored to your trial’s needs.
- Data Integration and Analytics: We break down data silos to provide a holistic, real-time view of your trial’s progress.
- Automated Quality Control: Our processes minimize manual effort and maximize data accuracy.
- Expert Support: Our team of data managers and biostatisticians ensures that your data is submission-ready, accelerating your path to regulatory approval.
By collaborating with CUREXBIO, organizations do not simply engage a service provider; instead, we form a strategic partnership aimed at reducing the duration of your development processes and improving the reliability of their data. CUREXBIO offers advanced Data Management solutions that can assist in transforming clinical trial data into a strategic asset. Our partnership is geared towards accelerating the delivery of vital therapies to patients. Organizations interested in expediting your clinical trial data management and enhancing your operational efficiency are encouraged to contact CUREXBIO for further collaboration.