When you take a medication, you expect it to heal you—- not cause unexpected harm. But even the most rigorously tested drugs can have side effects that only appear once thousands or millions of people start using them. This is where pharmacovigilance (PV) steps in —- the quiet, constant guardian ensuring medicines remain safe long after they reach the market.
At CUREX, we believe that an informed patient and a proactive healthcare provider form the best defense against drug-related harm. That’s why PV isn’t just part of our operations—- it’s at the heart of our mission.
Table of Contents
ToggleWhat exactly is Pharmacovigilance?
Pharmacovigilance is the science and practice of detecting, assessing, understanding, and preventing adverse effects or any drug-related problems.
Think of it as a safety net that operates continuously, ensuring that real-world experience with a drug is closely monitored and analyzed. PV involves:
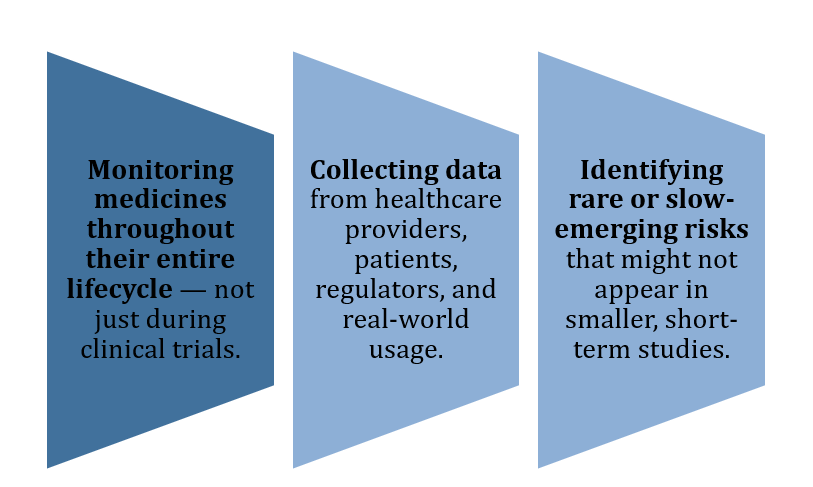
Figure 1: Pharmacovigilance in CUREX
“One patient’s experience can lead to safety measures that protect millions.”
Why Pharmacovigilance Matters More Than Ever
- Bridging the Clinical Trial Gap
-Clinical trials involve a limited number of participants under controlled conditions. PV extends this by tracking safety in diverse, everyday populations — elderly patients, pregnant women, people with multiple health conditions — uncovering risks that might otherwise go unnoticed. - Catching the Unpredictable
-Some adverse effects occur in less than 1 in 10,000 patients, or appear years after treatment. PV enables the long-term, large-scale observation needed to detect these rare events. - Ensuring Generic & Biosimilar Safety
-While generics match branded drugs chemically, differences in manufacturing can impact safety or efficacy. PV ensures these alternatives meet the same high safety standards. - Preventing Dangerous Drug Interactions
-By analyzing prescription trends, PV systems can flag hazardous combinations — like blood thinners with certain painkillers — before they cause harm.
The Evolution of PV: From Paper Reports to AI Insights
Pharmacovigilance has transformed from a slow, manual process into a data-driven global safety network:
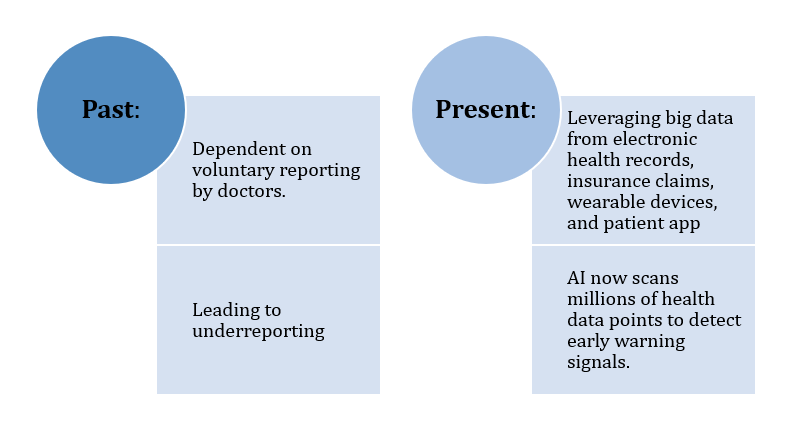
Figure 2: Evolution of Pharmacovigilance
At CUREX, we integrate anonymized, consent-based patient data into global PV databases, accelerating detection of potential risks and enabling faster safety interventions.
How Patients and Providers Play a Role
Pharmacovigilance thrives on community participation:
- Report Side Effects promptly : even if they seem minor. Small patterns can reveal big safety issues.
- Provide Complete Medical History: including allergies, other medications, and health conditions, to help analysts assess risks accurately.
- Myth: Only severe side effects are worth reporting.
- Truth: Mild or common side effects can be key to spotting trends and improving dosage recommendations.
CUREX’s Commitment to Excellence in Pharmacovigilance
We go beyond regulatory requirements, focusing on innovation and accessibility:
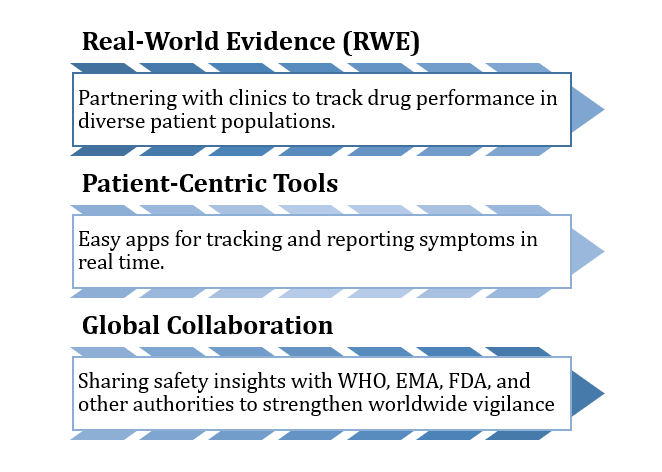
Figure 3: CUREX Commitment to Pharmacovigilance
The Bottom Line
Pharmacovigilance is more than just a regulatory checkbox >>> it’s a dynamic shield protecting public health. By turning data into actionable safety measures, PV ensures that today’s medical breakthroughs don’t become tomorrow’s public health crises.
At CUREX, we don’t just deliver treatments, we safeguard trust.
Stay Vigilant. Stay Safe.
Have a concern about your medication? Submit a report via our Safety Portal or speak directly with your CUREX care coordinator.
read also: Exploring Clinical Trials: A Comprehensive Guide to Medical Innovation