A well-designed clinical development plan (CDP) is crucial in drug development, guiding pharmaceutical companies from preclinical testing to regulatory approval. CUREXBIO understands the intricacies of this process and is dedicated to guiding companies through every step. A robust CDP minimizes risks, optimizes resources, and accelerates time-to-market, making it essential for start-ups and established firms in the drug development process. This blog highlights the process of creating and optimizing a clinical development plan, highlighting CUREXBIO’s expertise in clinical trials, pharmacokinetics, and regulatory strategy, and how they help clients refine these plans for maximum impact.
Table of Contents
ToggleUnderstanding the Clinical Development Plan
A clinical development plan is a comprehensive roadmap that integrates scientific, regulatory, and operational aspects to showcase a drug’s safety, efficacy, and quality.
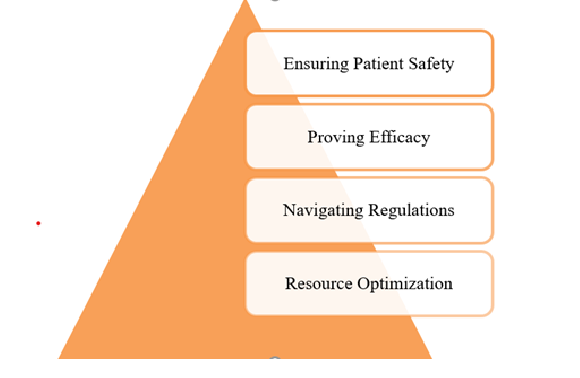
- Ensuring Patient Safety: Structured trials enable early identification of potential issues, facilitating timely interventions through systematic risk assessment.
- Proving Efficacy: It ensures the design of trials accurately measures therapeutic benefits, addressing any data gaps.
- Navigating Regulations: A robust CDP, in line with global standards like FDA and EMA, facilitates approvals and minimizes delays.
- Resource Optimization: The efficient allocation of time, budget, and personnel is a key factor in enhancing the overall success of a program.
Partnering with experts like CUREXBIO is crucial for development programs to avoid costly setbacks, regulatory hurdles, and failure, transforming potential pitfalls into strategic advantages.
How to Create a Clinical Development Plan
A CDP requires collaboration among clinical, regulatory, and statistical experts, and a step-by-step guide is provided for creating one.
- Target Identification: Start by pinpointing the disease or condition your drug aims to treat, considering unmet needs and market potential.
- Preclinical Testing: Conduct animal studies to evaluate safety, efficacy, and dosing. This data informs human trial designs and helps predict pharmacokinetics.
- Biomarker Identification (If Applicable): Select biomarkers for diagnostics, prognostics, pharmacodynamics, or safety monitoring to enhance trial precision.
- Phase I Trial Design: Define participant numbers, dose escalations, and endpoints. Collaborate with pharmacologists to model optimal dosing.
- Phase II Trial Design: Build on Phase I results to refine efficacy endpoints, and safety assessments, incorporating preclinical insights.
- Phase III Trial Design: Scale up with larger cohorts, focusing on endpoints that support labeling claims and demonstrate clear benefits.
- Data Analysis Plans: Establish statistical methods and quality controls to ensure robust, interpretable results.
- Regulatory Interactions: Outline filings, meetings, and strategies for engaging authorities like the FDA or EMA.
- Safety Monitoring: Develop protocols for ongoing vigilance during trials and post-approval.
Optimization of Clinical Development Plan
Optimization turns a good plan into a great one, reducing costs, enhancing flexibility, and improving outcomes. Follow these strategies:
- Complete Review: Analyze existing data, regulatory changes, and program goals to spot improvement areas.
- Expertise Consultation: Engage specialists in clinical, regulatory, and stats for tailored advice.
- Improve Target Population: Use demographics and disease data to focus on responsive subgroups.
- Maximize Trial Design: Adjust sample sizes, arms, and endpoints for better efficiency.
- Incorporate Real-World Evidence (RWE): Use external data to refine designs and expand insights.
- Enhance Regulatory Strategy: Collaborate with agencies on endpoints and benefit-risk assessments.
CUREXBIO Elevates Your Clinical Development Journey
Our team of experts in pharmacokinetics, toxicology, and regulatory affairs provides comprehensive support for clinical development plans, ensuring clients create and optimize successful plans.
- Preclinical and PK Expertise: We provide exploratory DMPK studies and pharmacokinetic profiling to establish a robust foundation, linking exposure data to safety outcomes for informed dosing.
- Trial Design and Execution: From Phase I to III, focus on protocol development, adaptive designs, and biomarker integration to ensure efficient and compliant trials.
- Regulatory and Safety Integration: We ensure safety through robust monitoring, and incorporating real-world relevance of RWE.
- Data-Driven Optimization: Utilizing advanced analytics and AI, we streamline plans based on interim data, reducing risks and expediting approvals.
We’re not just service providers, we’re collaborators in your quest for ground-breaking therapies. Contact us for our robust and futuristic services on bd@curexbio.com