At CUREX, we understand clinical trials are the driving force behind medical breakthroughs, transforming innovative ideas into life-changing treatments. This blog explores clinical trials, their types, phases, and key concepts like blinding, randomization, multi-phase trials, and pilot/feasibility studies, which are research studies designed to evaluate new ways to prevent, detect, or treat diseases.
Table of Contents
ToggleWhat Are Clinical Trials?
Clinical trials are research studies that test new approaches to prevent, detect, or treat diseases, forming the backbone of medical advancements. It examines:
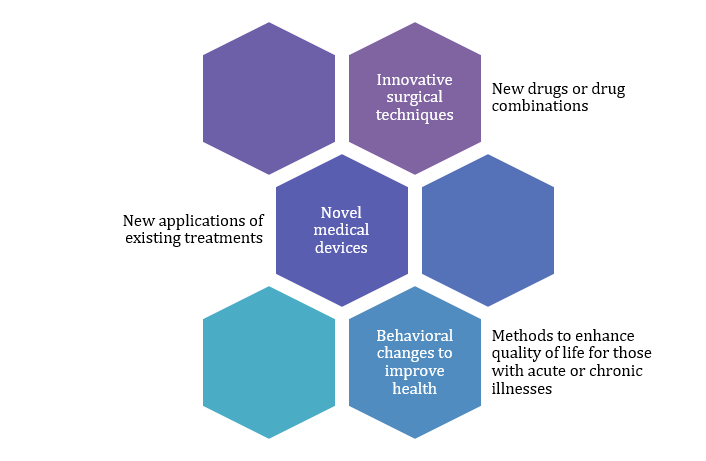
The initial aim of any clinical trial is to assess the safety and efficacy of interventions, with participants, including healthy volunteers and those with specific conditions, joining for various reasons, including science contribution, access to advanced treatments, and specialized care.
How Do Clinical Trials Work?
Clinical trials commence in the laboratory, testing new treatments or procedures using animal models, then progress to human trials, involving structured phases to gather data on safety, efficacy, and optimal use.
Each trial follows a protocol, a detailed plan that outlines:
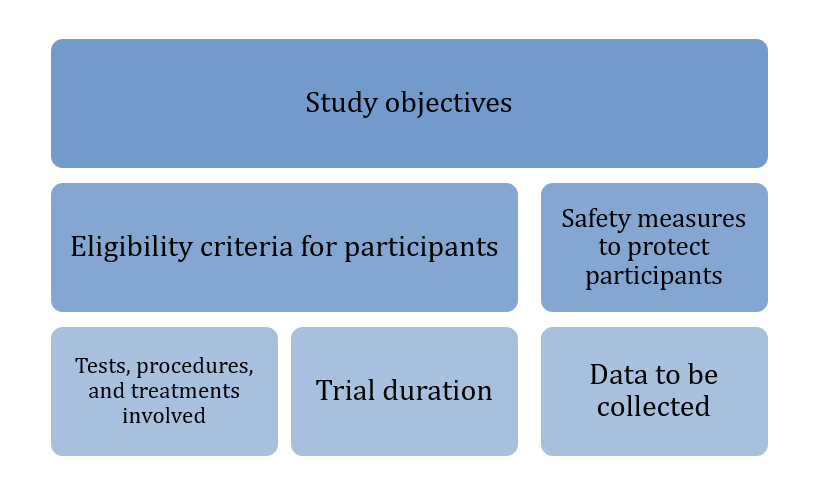
Figure 1: CUREX Protocol Outlines
A principal investigator (PI) leads the trial, with the research team closely monitoring participants’ health to ensure safety and evaluate the intervention’s impact.
Types of Clinical Trials
Clinical trials are divided into two main categories: observational studies and interventional studies.
Observational Studies
These studies observe participants without altering their treatments, aiming to identify patterns or risk factors. Types include:
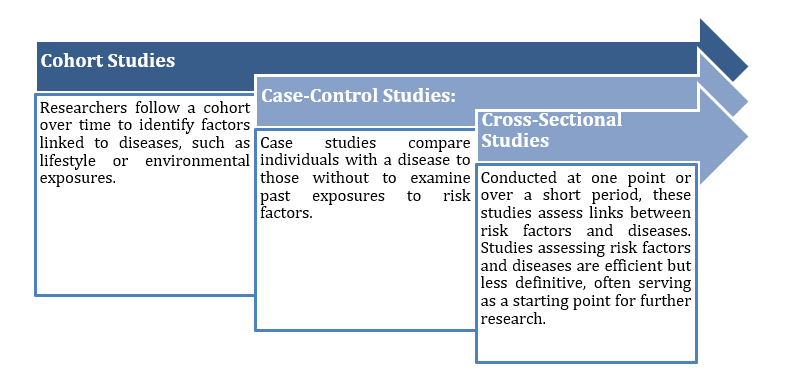
Figure 2: Observational study
Interventional Studies
These trials test specific interventions by assigning participants to treatment groups, often randomly, to compare outcomes. Types include:
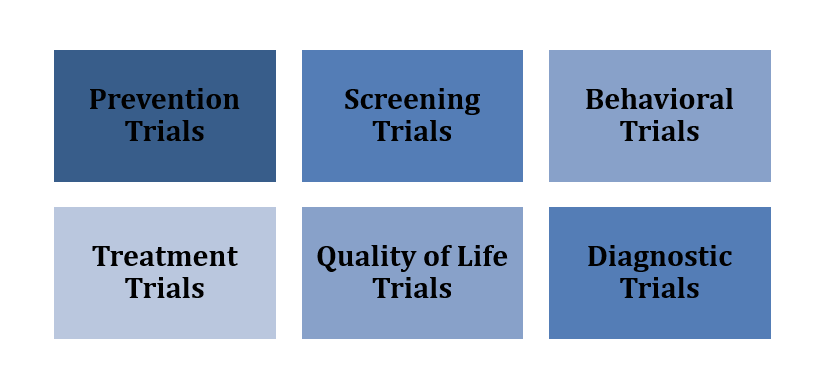
Figure 3:CUREX Interventional Study
Prevention Trials: These studies assess methods to prevent diseases, including vaccines, medications, and lifestyle changes, in healthy individuals and those at higher risk, like those with a cancer family history.
Screening Trials: The study evaluates the reliability and benefits of early disease detection methods for general or high-risk populations.
Treatment Trials: These explore new drugs, surgical techniques, radiotherapy methods, or devices to improve outcomes or quality of life for patients.
Behavioral Trials: These assess interventions to promote healthier behaviors, such as diet or exercise changes.
Quality of Life Trials (QoL): These focus on improving comfort and well-being for individuals with chronic conditions.
Diagnostic Trials: These compare or study tests for diagnosing specific diseases.
Advanced trial designs include:
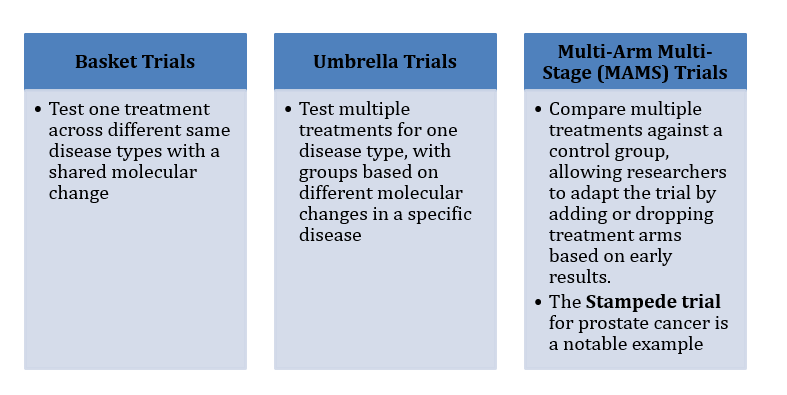
Figure 4: CUREX – Advanced Trial Design
Pilot and Feasibility Studies
Before large-scale trials, researchers may conduct pilot or feasibility studies to test the waters:
- Feasibility Studies: These assess whether a larger trial is practical, examining factors like participant recruitment, data collection, and analysis timelines. They don’t focus on treatment efficacy.
- Pilot Studies: Small-scale versions of the main study, these test whether all components work together and may provide preliminary data on efficacy. Results from pilot studies can sometimes be included in the main study’s findings.
Phases of Clinical Trials
Clinical trials progress through phases, each with a specific purpose:
- Phase 0: Early studies involving a small group (typically 10–20 people) receiving very low drug doses to confirm how a drug behaves in humans, based on lab findings. Participants may undergo extra tests (e.g., biopsies, scans) to assess drug behavior, with minimal side effects due to the low dose.
- Phase I: Tests a treatment in a small group (20–80 people), often those with advanced diseases who’ve exhausted other options. Goals include determining safe dosage, identifying side effects, and studying how the body processes the drug. Dose escalation is common, starting with low doses and increasing until the optimal dose is found. Frequent monitoring (e.g., blood tests, vital signs) is required.
- Phase II: Involves a larger group (100–300 people) to assess the treatment’s effectiveness and further evaluate safety. These trials often focus on specific cancer types and may compare the new treatment to an existing one or a placebo.
- Phase III: Large-scale trials (1,000–3,000 people) compare the new treatment to the standard treatment to confirm efficacy, monitor side effects, and assess quality of life. Most are randomized to ensure unbiased results.
- Phase IV: Conducted after a treatment is approved and available, these trials track long-term safety, rare side effects, and effectiveness in broader populations.
Trials Covering More Than One Phase
Some trials span multiple phases, such as Phase 1/2 or Phase 2/3, to streamline research. For example:
- A Phase 1/2 trial might test safety and dosage (Phase I) while beginning to assess efficacy (Phase II) in a single study.
- A Phase 2/3 trial combines efficacy testing with large-scale comparison to standard treatments, reducing the time needed to advance promising therapies.
These multi-phase trials are efficient, allowing researchers to gather more data without designing separate studies.
Randomization in Clinical Trials
Randomization is a critical process in many Phase II and most Phase III trials, where participants are assigned to treatment or control groups by chance, typically using a computer program. This minimizes bias by ensuring groups are comparable in factors like age, gender, or disease stage. For example:
- In a randomized controlled trial (RCT), one group receives the new treatment, while the control group receives the standard treatment or, if none exists, a placebo.
- Randomization ensures that differences in outcomes are due to the treatment, not pre-existing differences among participants.
Randomization enhances reliability. Without it, researchers might unintentionally assign less healthy patients to one group, skewing results. For instance, if sicker patients are placed in the control group, the new treatment might falsely appear more effective.
Blinding in Clinical Trials
Blinding (or masking) prevents bias by keeping participants, researchers, or both unaware of who receives which treatment:
- Single-Blind Trials: Participants don’t know whether they’re receiving the new treatment, standard treatment, or a placebo, but researchers do. This prevents participants’ expectations from influencing outcomes.
- Double-Blind Trials: Neither participant nor researchers know who’s receiving which treatment. A computer assigns code numbers to participants, and treatments are labell only with these codes. Only the pharmacist knows the assignments, ensuring unbiased data collection. In emergencies, researchers can unblind a participant’s treatment to ensure safety.
Blinding is crucial for objective results. For example, if participants know they’re receiving a new drug, their optimism might influence reported outcomes (a placebo effect). Double-blind trials are consider the gold standard for minimizing bias.
Placebos in Clinical Trials
A placebo is an inactive treatment (e.g., a sugar pill or saline injection) designed to resemble the active treatment. Placebos are use in trials where no standard treatment exists, allowing researchers to compare the new treatment’s effects against no treatment. Ethical guidelines prohibit placebos when effective treatments are available, ensuring participants aren’t deprive of necessary care.
Why Clinical Trials Matter
Clinical trials are the gateway to medical innovation, turning lab discoveries into safe, effective treatments. They offer patients access to cutting-edge therapies and provide critical data to improve healthcare globally. At CUREX, we’re dedicate to supporting clinical research that transforms lives.
Whether you’re a potential participant or simply curious and understanding clinical trials empowers you to engage with the future of medicine. Stay connected with CUREX for the latest updates on groundbreaking research and opportunities to contribute to medical progress!
read also: Unleash the Power of Bioanalytical Testing and Method Validation with CUREX





