Clinical data management is vital in clinical trials, guaranteeing that data is accurate, reliable, and prepared for analysis and regulatory submission. With the increasing complexity and data volume from multiple sources, effective clinical trial data management is crucial for producing high-quality evidence and expediting drug development.
Table of Contents
ToggleClinical Data Management in Clinical Trials
Clinical data management (CDM) refers to the processes, tools, and personnel involved in the collection, cleaning, validation, and management of data during a clinical trial. It connects raw data entry to final analysis while ensuring adherence to regulations such as Good Clinical Practice (GCP), FDA/EMA guidelines, and 21 CFR Part 11. The aim is to generate reliable and trustworthy datasets that ensure dependable conclusions regarding the safety and effectiveness of a treatment.
Key Roles in Clinical Data Management
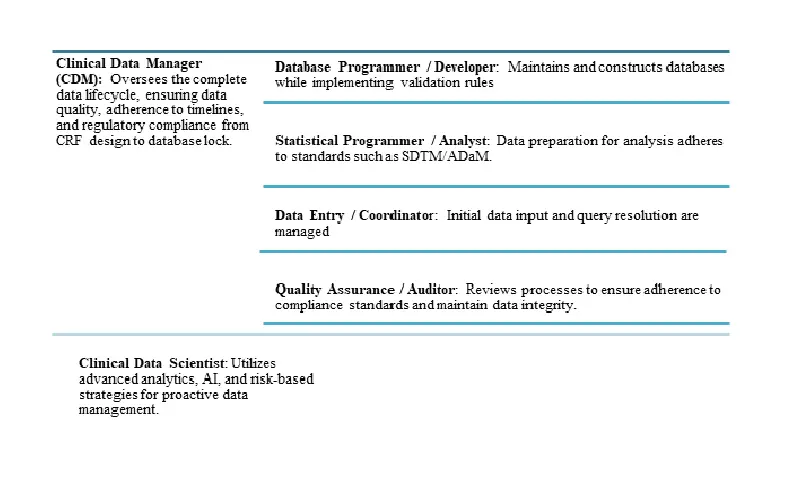
These roles necessitate collaboration with biostatisticians, clinical operations, and regulatory teams.
Arrays of Essential Skills for Clinical Data Management Professionals
Success in clinical trial data management requires a combination of technical, regulatory, and soft skills.
- Strong knowledge of regulatory standards, including GCP, ICH guidelines, and CDISC.
- Proficiency in handling, validating, and cleaning data is important.
- Expertise in electronic data capture (EDC) systems, Statistical analysis system (SAS), Structured query language (SQL), and Python is used for programming and analytics.
- Expertise in EDC systems, SAS, SQL, and Python is essential for programming and analytics.
- Effective communication skills are significant for resolving queries and fostering teamwork across different functions within an organization.
- Understanding CDISC standards involves knowledge of key components such as SDTM for tabulating data, ADaM for analysis, and CDASH for data collection.
As clinical data science advances, expertise in AI, machine learning, and data visualization becomes increasingly important.
CDMS Tools and Electronic Data Capture (EDC) Systems
A clinical data management system (CDMS) centralizes data handling in clinical trials, often incorporating electronic data capture (EDC) for real-time, paperless data collection through electronic case report forms (eCRFs).
Popular CDMS and EDC tools have gained prevalence in recent years.
- Veeva Vault EDC — Cloud-native, fast study builds, and remote monitoring support –
- Medidata Rave EDC Industry leader for large-scale trials with robust integration.
- Oracle Clinical One — Unified platform for EDC, randomization, and supplies.
- Clinion EDC — AI/ML-enhanced for rapid deployment and unified data management.
- Others like Viedoc, OpenClinica, Medrio, and Castor — offering user-friendly, compliant options for various trial sizes.
These tools provide real-time data validation, query management, audit trails, and integration with other eClinical systems such as CTMS and IRT.
Understanding CDISC Standards for Data Standardization
CDISC (Clinical Data Interchange Standards Consortium) standards foster data consistency, interoperability, and expedite regulatory review globally. Key components of CDISC are crucial to this process.
- CDASH— For standardized data collection.
- SDTM (Study Data Tabulation Model) — Organizes raw data for submission.
- -ADaM (Analysis Data Model) — Prepares analysis-ready datasets.
- ODM — For data exchange and metadata.
Adhering to CDISC standards reduces errors, enables data pooling across studies, and simplifies submissions to regulatory agencies such as the FDA and EMA, which require these standards for numerous submissions.
Future Trends in Clinical Data Management
The landscape of clinical data management is rapidly evolving, driven by technology and regulatory shifts.
- AI and automation are transforming cleaning processes by shifting focus from reactive cleaning to proactive risk-based quality management (RBQM), which utilizes predictive analytics and automated query detection.
- Integration of Real-World Data (RWD) and wearables involves using data from devices and mobile applications alongside decentralized trials to enhance patient-centric approaches.
- Cloud-based and unified platforms facilitate seamless data integration, scalability, and remote access.
- Advanced Data Science highlights the transition from traditional Clinical Data Management (CDM) to clinical data science, focusing on the integration of machine learning and the importance of real-time insights.
- Blockchain technology enhances security by improving data integrity, traceability, and privacy.
- Decentralized and Continuous Trials supports hybrid models with better interoperability
These trends promise faster trials, higher data quality, and more efficient regulatory pathways.
CurexBio offers extensive clinical data management services for comprehensive clinical trial support. Our expertise includes clinical trial data management, data cleaning, validation, and adherence to global standards such as CDISC, ensuring data integrity throughout Phase I–IV studies. CurexBio also integrates with modern CDMS tools, providing risk-based monitoring and pharmacovigilance to deliver efficient, high-quality clinical data management in trials.
Robust clinical data management is essential for successful clinical research. Utilizing skilled professionals, advanced CDMS tools, CDISC standards, and emerging technologies enables organizations to manage complexity and provide reliable real-world evidence. Partnering with experienced providers like CurexBio can significantly enhance trial planning. Contact us today by email at bd@curexbio.com!!





