The clinical development landscape is experiencing significant changes, moving away from traditional, linear, and fixed Clinical Trials designs. These conventional methods are increasingly seen as rigid, inefficient, and not fully capable of addressing the complexities inherent in modern medicine. In response, adaptive clinical trial designs have emerged as a transformative strategy, enabling planned and data-driven adjustments throughout the trial process.
To provide a comprehensive framework for this innovative approach, the International Council for Harmonization (ICH) introduced the pivotal ICH E20 guideline in 2023. This guideline aims to establish global clarity and standards for adaptive trials, paving the way for more flexible and responsive clinical research methods.
In this context, we aims to clarify the ICH E20 guideline, highlight the advantages of adaptive designs, and CurexBio is adeptly prepared to navigate these advanced development pathways to aid in the progress of breakthrough therapies.
Table of Contents
ToggleWhat is ICH E20 and Why Does It Matter?
ICH E20, titled “Adaptive Designs for Clinical Trials,” is the inaugural internationally harmonized guideline focused on the planning and execution of adaptive trials. Its publication represents a significant milestone, reflecting global regulatory acceptance and offering a comprehensive framework for sponsors involved in such clinical studies.

Figure 1: Core Mission of ICH E20
Common Types of Adaptive Designs include:
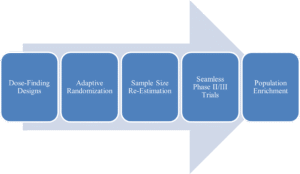
Figure 2: Types of Adaptive clinical trials
- Dose-Finding Designs: Efficiently identify the optimal therapeutic dose by allocating more patients to promising dose levels and away from toxic or ineffective ones.
- Adaptive Randomization: Adjust the randomization probability to favor the treatment arm showing better interim results, offering ethical benefits to participants.
- Sample Size Re-Estimation: Recalculate the required number of patients at an interim analysis based on observed variability or treatment effect, ensuring the trial is neither under- nor over-powered.
- Population Enrichment: Refine the target patient population during the trial to focus on those most likely to respond (e.g., based on a biomarker).
- Seamless Phase II/III Trials: Combine proof-of-concept and confirmatory stages into a single, continuous trial, saving significant time and resources.
Advantages of Adaptive clinical trials:
- Faster Development: Get effective therapies to patients sooner.
- Higher Probability of Success: Make informed decisions mid-trial to focus resources on winning strategies.
- Cost Efficiency: Reduce the number of patients exposed to ineffective treatments and optimize resource allocation.
- Enhanced Ethical Standing: Treat trial participants more intelligently and compassionately.
The Challenge: Complexity and Regulatory Scrutiny
The power of adaptive designs comes with complexity. Their success hinges on:
- Meticulous Pre-Planning: Every potential adaptation must be exhaustively detailed in the protocol and statistical analysis plan *before the trial begins*.
- Advanced Statistical Expertise: Requiring sophisticated simulation and modeling to control Type I error and avoid operational bias.
- Robust Operational Infrastructure: Dynamic randomization, real-time data capture, and stringent interim analysis processes with firewalls.
- Proactive Regulatory Engagement: Clear communication and alignment with health authorities is non-negotiable.
Curex Bio Empowers Your Adaptive Design Strategy
A poorly planned adaptive trial may result in invalid outcomes, regulatory non-acceptance, and squandered investments. In such scenarios, having a specialized partner like CurexBio proves to be essential.
At CurexBio, we focus on providing a comprehensive strategic partnership aimed at maximizing the effectiveness of ICH E20 and adaptive designs within clinical programs. Our integrated approach enhances collaboration and supports the overall success of clinical initiatives.
- Strategic Design & Simulation:
Our team of biostatisticians and clinical scientists collaborates with clients to assess the appropriateness of adaptive design for their objectives. We explore various design options, quantify essential operating characteristics such as power and sample size distributions, and develop a comprehensive, pre-specified adaptation plan that meets regulatory standards and instills confidence.
- Regulatory Pathway Mastery:
We develop thorough documentation in accordance with ICH E20 standards, which encompasses a detailed adaptive design charter. Our regulatory affairs team is instrumental in preparing for and facilitating essential interactions with the FDA, EMA, and other regulatory agencies, ensuring that all processes are aligned right from the beginning.
- Technology-Enabled Execution:
We implement and manage integrated technology platforms that facilitate real-time data collection, centralized monitoring, and dynamic randomization processes. These measures are essential for ensuring data integrity and preventing operational bias during critical adaptation points.
- Operational Excellence & Risk Mitigation:
Our clinical operations team specializes in the distinct requirements of adaptive trials, creating comprehensive playbooks for a variety of adaptation scenarios. We take a proactive approach in identifying and mitigating risks, which allows for the seamless execution of complex trials across multiple global sites.
Why Partner with Curex Bio?
- Deep Knowledge Our team includes veterans who have successfully designed, defended, and executed adaptive trials across therapeutic areas.
- Flexible Partnership: We integrate seamlessly with your team, offering flexibility and transparency.
- Focus on Your Success: We are committed to making your trial a model of efficiency and scientific integrity, accelerating the path to approval.
Ready to design a smarter, faster clinical trial? Contact CurexBio today to explore how our adaptive design services can de-risk and accelerate your path to market.